Let know about Accreditation
Accreditation is the process in which an accreditation body is endowed with business ethics, utmost integrity, unconditional credibility, and maintaining absolute neutrality is given to the responsibility and authority to undertake certification and assessment of a firm in the most appropriate manner.
Globalization process has brought great changes in the way trade is done in between the buyer and the seller. It has cut across boundaries and simplified the process by incorporating certain new process such as third-party. These third party auditors take the role of responsible advisors by assisting the buyer and the seller through the process of certification that is neutral and fair.
Accreditation is defined by International standards of organization as “third party attestation” related to a conformity assessment conveying formal demonstration of its competence to carry out specific conformity assessment tasks (ISO / IEC 17021)

Accreditation Benefits
Many governing bodies and government organizations prove their assistance for accreditation by stating:
“Accreditation is important for the proper functioning of a translucent and quality-focused market.”
The particular advantages of attaining accreditation from the ASIB can be summarized as follows:
Demonstration of your proficiency
Evidence of your self-sufficiency
Verification of your ethics and professionalism
accomplish global recognition for your organization
Upkeep of your overall performance by normal monitoring by an impartial third party

Standard Description
ISO/IEC 17020 – General criteria for the operation of various types of bodies performing inspection
ISO/IEC 17021 – Conformity assessment – Requirements for bodies providing audit and certification of management systems
ISO/IEC 17024 – General requirements for bodies operating certification of persons
ISO/IEC 17025 – General requirements for the competence of testing and calibration laboratories
ISO/IEC 17065 – General requirements for bodies certifying products, processes and services
ISO 15189:2012 – Specifies requirements for quality and competence in medical laboratories
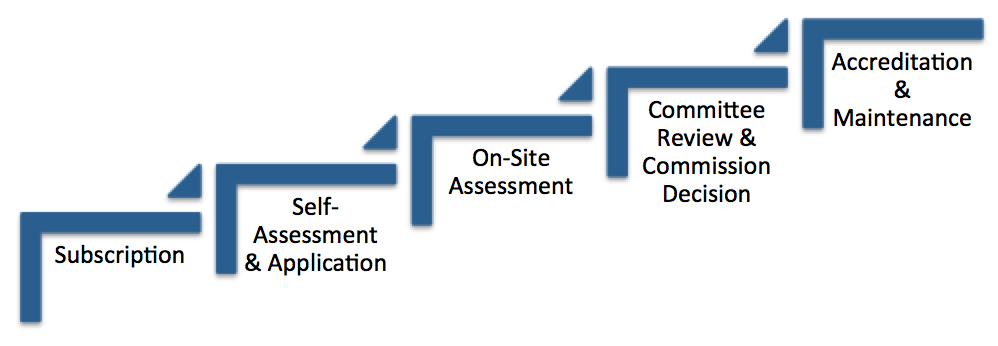
Accreditation Process
The main elements of the accreditation process are:
Self-assessment by the provider of the service against the Quality Standards
Submission of an application for accreditation of a commencing service
Submission of an application for re-accreditation of an accredited service or recommencing service
Assessment by a team of registered quality assessors at a site audit and development of a site audit report
Development of a performance assessment report outlining compliance with the Quality Standards
A decision about the service’s accreditation by a decision-maker (delegate of the Commissioner)
Issue of an accreditation certificate, the certificate of accreditation may be displayed at the accredited service
Publication of the performance report and accreditation decision on this website
Monitoring the quality of care and service at the service and managing any non-compliance with the Quality Standards